Thermoplastic vs. Thermosetting Polymers Explained
The principal differences between the two fundamental polymer systems used in the advanced composites industry, thermoplastic and thermosetting polymers, are based on how they are formed to make a solid polymer resin and the presence, or lack of, cross-linking molecular groups connecting the long chain polymer repeating molecules.


Illustration of the lack of, or presence of, long chain polymer cross links can be shown as follows, where the figure on the left represents a thermoplastic polymer and the one on the right represents a thermosetting polymer.
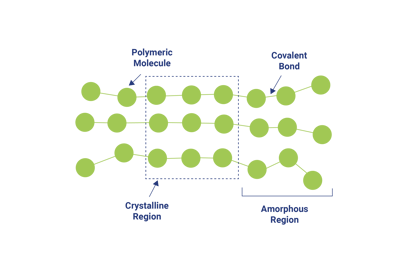
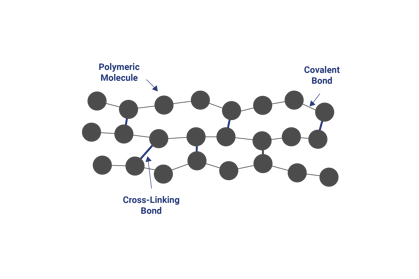
Basically, thermosetting polymers have covalent or ionic bonds between the cross-linking molecule and the long chain polymer molecules. These covalent or ionic bonds form a strong and irreversible attachment to the long chain polymer.